Emma Fields, MD |Weston Stover, MD | Dorin Todor, Ph.D. | Brian Kaplan, MD | Ross Taylor, MD
BACKGROUND
Pancreatic cancer remains one of the most aggressive tumors with low rates of long-term survival.1 The cause of the dismal prognosis is multifactorial and can be attributed to patients often presenting for clinical evaluation very late in the natural history of the disease and the aggressive nature of the tumor with early vascular and neural invasion.1,2 Surgical resection remains the only curative option, however, of the 50% of patients who present with localized disease, only 10-20% of patients are resectable at diagnosis. Resectability is classified into three categories: resectable, borderline resectable (BR), and unresectable. There have been a number of definitions and therefore subjectivity as to what determines BR, but the most recent NCCN guidelines define it as tumor abutment less than or equal to 180 degrees of a major peri-pancreatic vessel.1,3 Patients with concern for vascular involvement benefit from neoadjuvant chemotherapy and radiotherapy to optimize the surgical outcomes.2–4
The current standard of care for borderline-resectable pancreatic cancer is centered on the use of neoadjuvant therapy followed by surgical resection. There are various acceptable approaches for neoadjuvant therapy including chemotherapy, chemotherapy followed by radiation with either SBRT or chemoradiation and other iterations of these modalities. Despite this multi-modality approach, achievement of posterior/retroperitoneal negative margins (R0) is technically challenging and many patients will have microscopic disease (R1) at the surgical margin which is associated with increased local recurrences and poorer overall survival. 5,6 The rates of microscopic margin involvement reported in the literature vary markedly, from as low as 16% to greater than 75%, and are generally thought to be underreported as there is no standardized method of evaluating or defining resection margins7–9. It is currently unknown what constitutes an adequate margin, but retrospective data suggest that margins of at least 1 millimeter provide improved patient outcomes when compared to those with close or positive resection margins.9–12
For patients with close or positive margins after surgical resection, there is no consensus for the role of an additional radiation boost. In this situation it is very challenging to boost the region of concern as the anatomy is distorted, the surgical clips can be unreliable, and the close proximity of the already radiated luminal bowel. Intraoperative radiotherapy (IORT) has emerged as an attractive option allowing for targeted radiation doses to high-risk regions of local failure. In pancreatic cancer, IORT has been extensively studied given the complex anatomical region and high risk for positive resection margins and may offer a viable option to improve patient outcomes.13 However, standard IORT requires a linear accelerator in the operative suites as well as installation of appropriate shielding and is thus limited by cost and equipment requirements. The CivaSheet® (sheet) is a form of brachy-IORT developed to capture the benefits of the IORT technique, whilst providing brachy-specific advantages over traditional IORT.
CivaSheet Physical Properties and Dosimetry
It is an FDA-cleared implantable matrix of uni-directional planar low-dose-rate (LDR) Palladium-103 (Pd-103) radiation sources encapsulated in an organic polymer and embedded within a grid that consists of a flexible encapsulated substrate.13 Each source is shielded on one side by a gold disk to attenuate the dose to approximately one-tenth of the total dose, making the radiation distribution directional to target only the high-risk margin (Figure 1)14–16 The sheet is currently manufactured in two sizes that can be cut to a customized size in the operating room: a small 5 x 10 cm sheet containing of 72 sources and a large 5 x 15 cm sheet containing 108 sources.
CivaSheet Clinical Work Flow
There is a general protocol and workflow for institutions investigating the use of this novel device for intraoperative radiotherapy (Figure 2). At initial diagnosis, patients with borderline resectable pancreatic adenocarcinoma are reviewed by the multidisciplinary team. Our standard approach is to proceed with neoadjuvant chemotherapy for 4-6 months followed by imaging. At that time if there is no evidence of metastatic disease, the patient receives focal radiotherapy. Based on serial imaging, the multidisciplinary team then determines the resectability and risk of close or positive margins. Once a patient is identified at high risk for a R1 resection, a pre-plan is made by the radiation oncology team in advance of the scheduled surgery to determine the size of the sheet, prescription dose, and air kerma strength that will be required. The Civasheet order is placed at least 2 weeks prior to the proposed date of surgery with the goal of the sheet arriving 2 days prior to the implant for source calibration.
On the day of surgery, after the resection is completed, the radiation oncology team enters the operating room to collaborate on the sheet placement. The area of highest risk for close margins is identified by the surgeon and confirmed by the radiation oncologist. With the physicist and radiation oncologist present, the surgeon measures the agreed-upon area within the resection bed using a piece of telfa, cuts the device to size, and secures it to the retroperitoneum with sutures and fibrin sealant. Importantly, this can be done via standard open or laparoscopic approaches. Unlike most of radiotherapy, where the target is identified by some form of imaging, here the target is identified visually and the device secured in place before any other surgical manipulation takes place, thus eliminating the need for increased margins to accommodate placement uncertainty.
Prior to discharge of the patient, CT scans are obtained in the radiation oncology department to localize the dots and retrieve the post-implant dosimetry (Figure 3). Subsequent follow-up CT imaging is obtained at 30 and 60 days to evaluate any source migration or complications.
CivaSheet Calibration and Dosing
The air kerma strength for the implant day is ordered based on a nomogram that outputs the total air kerma strength needed for a given prescription dose and depth of the prescription. Prescription dose is given by specifying either D90 or D95 levels for a generic volume (the surface of the mesh x prescription depth). The dose is typically prescribed to 5 mm, and doses in this setting have ranged from 30.8 to 50.4 Gy in the Phase 1 trial, and the higher dose is now the primary candidate in the Phase 2 expansion.16
Once the device arrives, per the “Report of the AAPM Low Energy Brachytherapy Source Calibration Working Group,” a minimum of 10% of the number of sources ordered are calibrated using a standard brachytherapy well chamber. The calibration sources are received as separate CivaDots, specially designed for placement in a custom well chamber insert. Given the physical properties of Pd-103, the device will actively deliver radiation to the site for approximately two months (half-life =17 days).
Clinical Results
Early data suggests that the use of Civasheet as a form of IORT for pancreatic cancer is safe, well-tolerated, and is relatively simple to implement. A first report of the feasibility of this device has recently been published including an initial cohort of 11 patients with pancreatic cancer. Of these patients, 1 was resectable and went straight to surgery and the other 10 were borderline resectable and received neoadjuvant chemotherapy followed by either chemoradiation or stereotactic body radiotherapy and had subsequent resection with sheet placement at the time of surgery. On average, the sheet placement added 15 minutes to the overall procedural time with no procedural or postoperative complications attributable to the sheet placement.
On final pathology, retroperitoneal margins were confirmed negative in all cases (0.3 – 20 mm). CT simulation within 7 days postoperatively confirmed placement and was used to evaluate the dose. The median prescription physical dose was 37.8 Gy (30.8 – 50.4 Gy) or 25Gy EQD2 (15-35Gy EQD2).
At a median follow-up of 13 months, 64% (7) of patients were alive and 55% (6) were disease-free. Of the six patients alive without disease, three had retroperitoneal margins of 3 mm or less and another patient had a negative margin with unreported distance. Three of the four patients who developed local recurrence had ypT3 disease. In two of these cases, recurrences were outside of the target volume of the sheet and in one case outside imaging was not available to assess the location of recurrence. In the one case of recurrence with ypT1 disease, the recurrence appeared to begin well medial to the sheet at 5 months but grew to be adjacent to the sheet.16
There is currently a prospective multi-institutional Phase I/II trial (NCT02843945) accruing to further determine the safety and efficacy of the Civasheet with the primary endpoint of monitoring for any adverse radiation toxicity effects at one year. Secondary endpoints include local recurrence rates at 1 year and overall survival at 2 years. At the time of this writing, CivaTech Oncology reports there are currently 6 centers actively recruiting patients to this study with 31 patients on trial.
Advantages of CivaSheet for IORT in Pancreatic Cancer
The initial data for the CivaSheet appears to be promising as it maintains the basic principles of IORT in the setting of pancreatic cancer to provide durable intraoperative doses of radiation and preferentially spare nearby organs at risk. Additionally, it has other advantages over electron beam, superficial x-ray, high- or low-dose-rate IORT options. There is no need for increased infrastructure with costly investments including after loaders, linear accelerators, or shielded operating rooms.14,16 External beam IORT machines are large and customization limitations of currently available electron cones make the targeting of complex anatomic surfaces difficult.17 The deployment of the sheet only requires standard surgical techniques and does not significantly increase time in the operating room. The proper orientation of the unidirectional sources is easy to visualize at the time of implantation given the gold shielding (“gold is cold”) as well as on postoperative CT imaging which allows for in vivo dosimetry calculations. Previously developed Mesh LDR implantable devices can be difficult to bend and contour around irregular surfaces leading to alterations in isodose geometry leading to large inhomogeneities, however, the sheet’s unique polymer encapsulation of the Pd-103 sources embedded within the membrane allows for maintenance of spatial orientation and delivery of the radiation in accordance with the predetermined geometry17. The unique unidirectional design of the CivaDots with the gold shielding significantly attenuates the dose-limiting toxicity to nearby dose-limiting organs at risk. To this date, there have been no reported toxicity or complications directly related to the device including complications from surgery or migration of sources.16
Limitations of CivaSheet for IORT in Pancreatic Cancer
Despite these advantages, there are a number of limitations to consider. As this is a new therapy, there are limited reported data to support its efficacy and safety at this time. The study by Dault et al. had favorable clinical results with no toxicities attributable to the sheet, but it is a small retrospective series with a short median follow-up.
One of the most concerning aspects of an implanted brachytherapy device is the risk of seed migration or shifts in the position of the dots. Given the complexity of the procedure to place the sheet and the bowel reconstruction required afterward, it would be incredibly challenging to retrieve sources. Additionally, it is possible migration could happen without clinical awareness. In theory, the biodegradable substrate on which the sources are placed is supposed to preserve the geometry for at least 90 days. Furthermore, in the event of implant migration, unlike HDR delivery of treatment, LDR therapy does not allow for changing dwell times or real-time planning to potentially mitigate errors in placement. Presently, there have been no reported postoperative infections related to the implant, however, implantation of any foreign device increases the risk of infection and these patients could be at higher risk for postoperative complications. We will await the data from the larger prospective Phase 2 clinical trial for more clinical outcomes.
Another challenge is that the logistical planning and implantation of the sheet requires coordination between multiple departments, personnel, and the manufacturer. In practice, the use of this device requires buy-in from the surgical oncology team, as they are the end-users and the providers trusted by the patient to perform the procedure.
Dose specification depends on multiple clinical aspects including defining of the reference dose and depth, the calculation geometry, as well as the actual desired dose, and these specifications are the basis of source ordering decisions. An order for the device must be placed weeks in advance of the planned surgery and any deviation or unforeseen delay may lead to inadequate source strength or potentially an unusable product. A dosimetric study has shown that nonsquare and smaller (<6 x 6cm) sheets might require higher source strengths compared to their square and larger counterparts, so anticipating expected size, geometry, and dimensions needs to be considered prior to ordering, which can be difficult to anticipate15. Like all new therapies, hospitals need reimbursement in order to treat patients, but while inpatient reimbursement codes are being applied for, the company is dedicated to providing access to pancreatic cancer patients.
Conclusions
The Civasheet offers a solution to a well-characterized limitation in the treatment of borderline resectable pancreatic cancer by allowing for focal high dose radiation to regions at risk for close/positive margins. There are potentially other options including additional chemotherapy, external beam radiotherapy boost, or re-irradiation SBRT to address this limitation although they are not well established in the literature. Early feasibility and safety data are promising. The sheet can be used in conjunction with the current multimodality approach of neoadjuvant chemotherapy and external beam radiation followed by surgical resection. Given the ease of use, it is amenable to implementation in most institutions without significant upfront cost, additional construction, or delay. We are eagerly awaiting prospective data to further elucidate the potential role for this novel device in this patient population that desperately needs breakthrough treatment options.
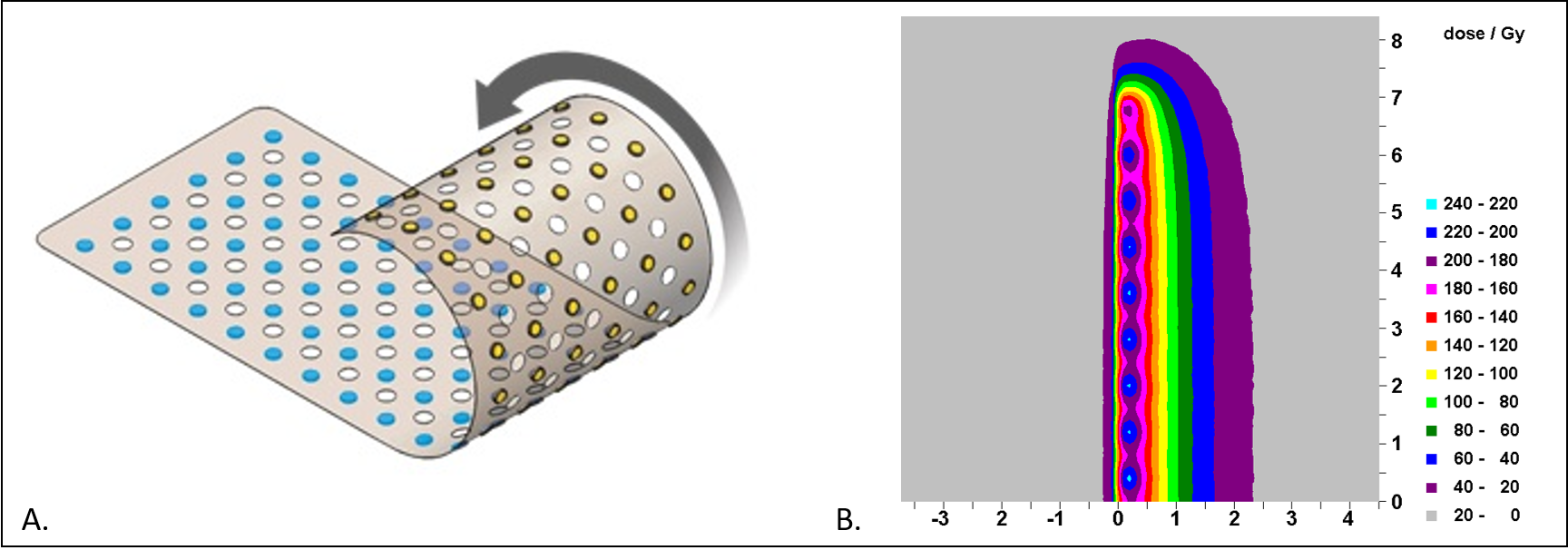
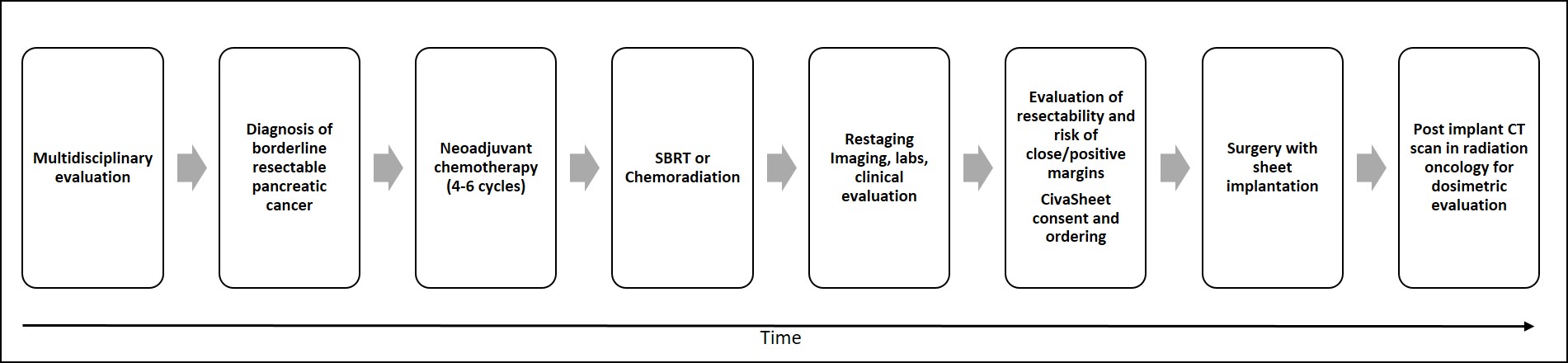
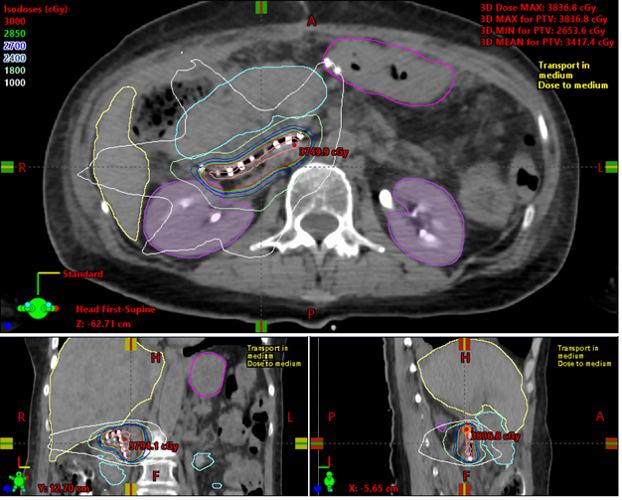
Figure Legends:
Figure 1. A. The CivaSheet contains a discrete array of palladium 103 sources (blue) with gold shielding on the reverse side set within a bioabsorbable polymer. Each CivaDot is spaced 8 mm apart. B. Low dose rate dosimetric characteristics of the CivaSheet. The dose is typically prescribed to 5 mm depth.
Figure 2. Treatment timeline with CivaSheet implantation for borderline resectable pancreatic cancer
Figure 3. Example of post-operative dosimetric calculation of CivaSheet.
References
1. Lekka K, Tzitzi E, Giakoustidis A, Papadopoulos V, Giakoustidis D. Contemporary management of borderline resectable pancreatic ductal adenocarcinoma. Ann Hepato-Biliary-Pancreatic Surg. 2019;23(2):97. doi:10.14701/AHBPS.2019.23.2.97
2. Denbo JW, Fleming JB. Definition and Management of Borderline Resectable Pancreatic Cancer. Surg Clin North Am. 2016;96(6):1337-1350. doi:10.1016/J.SUC.2016.07.008
3. Toesca DAS, Koong AJ, Poultsides GA, et al. Management of Borderline Resectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2018;100(5):1155-1174.
4. Kommalapati A, Tella SH, Goyal G, Ma WW, Mahipal A. Contemporary management of localized resectable pancreatic cancer. Cancers (Basel). 2018;10(1). doi:10.3390/CANCERS10010024
5. Gnerlich JL, Luka SR, Deshpande AD, et al. Microscopic Margins and Patterns of Treatment Failure in Resected Pancreatic Adenocarcinoma. Arch Surg. 2012;147(8):753-760. doi:10.1001/ARCHSURG.2012.1126
6. A W, S T, IN F, et al. Resectable adenocarcinomas in the pancreatic head: the retroperitoneal resection margin is an independent prognostic factor. BMC Cancer. 2008;8. doi:10.1186/1471-2407-8-5
7. Verbeke CS. Resection margins and R1 rates in pancreatic cancer – are we there yet? Histopathology. 2008;52(7):787-796. doi:10.1111/J.1365-2559.2007.02935.X
8. Esposito I, Kleeff J, Bergmann F, et al. Most Pancreatic Cancer Resections are R1 Resections. Ann Surg Oncol 2008 156. 2008;15(6):1651-1660. doi:10.1245/S10434-008-9839-8
9. Kim KS, Kwon J, Kim K, Chie EK. Impact of resection margin distance on survival of pancreatic cancer: A systematic review and meta-analysis. Cancer Res Treat. 2017;49(3):824-833. doi:10.4143/CRT.2016.336
10. Chandrasegaram MD, Goldstein D, Simes J, et al. Meta-analysis of radical resection rates and margin assessment in pancreatic cancer. Br J Surg. 2015;102(12):1459-1472. doi:10.1002/BJS.9892
11. Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, Version 2.2021. JNCCN J Natl Compr Cancer Netw. 2021;19(4):439-457. doi:10.6004/JNCCN.2021.0017
12. Tuli R, Osipov A, Rutgers JK, et al. Margin Distance in Resected Pancreatic Cancer Independently Predicts Survival: Implications for Adjuvant Radiation Therapy Using Pelvic Bone Marrow Hounsfield Units to Predict Cytopenia During Anal Cancer Chemoradiation E214. Int J Radiat Oncol Biol Phys. Published online 2016. doi:10.1016/j.ijrobp.2016.06.1128
13. A directional 103Pd brachytherapy device: Dosimetric characterization and practical aspects for clinical use | Elsevier Enhanced Reader. Accessed August 23, 2021. https://reader.elsevier.com/reader/sd/pii/S1538472116306110?token=5345C2587DCE7E874ECB7EA1EFEEABF8E6746A78C12D749E365E4FE84F8DFF68B2073CAFF19DE42A657C61EB638AFF53&originRegion=us-east-1&originCreation=20210823115422
14. Cohen GN, Episcopia K, Lim SB, et al. Intraoperative implantation of a mesh of directional palladium sources (CivaSheet): Dosimetry verification, clinical commissioning, dose specification, and preliminary experience. Brachytherapy. 2017;16(6):1257-1264. doi:10.1016/J.BRACHY.2017.07.010
15. GN C, K E, SB L, et al. Intraoperative implantation of a mesh of directional palladium sources (CivaSheet): Dosimetry verification, clinical commissioning, dose specification, and preliminary experience. Brachytherapy. 2017;16(6):1257-1264. doi:10.1016/J.BRACHY.2017.07.010
16. Dault JB, Todor D, Kaplan BJ, Myers JL, Fields EC. First report on the feasibility of a permanently implantable uni-directional planar low dose rate brachytherapy sheet for patients with resectable or borderline resectable pancreatic cancer. Brachytherapy. 2021;20(1):207-217. doi:10.1016/j.brachy.2020.08.010
17. Seneviratne D, Mclaughlin C, Todor Ph.D. D, Kaplan B, Fields EC. The CivaSheet: The new frontier of intraoperative radiation therapy or a pricier alternative to LDR brachytherapy? Published online 2017. doi:10.1016/j.adro.2017.10.005